The Gigafactory: Death In The Desert
The Gigafactory: Death In The Desert
(Click Here For PDF) This report presented to the FBI, The GAO, The EPA and the U.S. Congress
When reporter Andy Barron was attacked and beat-up, by Elon Musk’s hench-men, at the construction site for the Nevada Tesla “Gigafactory”, the toxic secret about Tesla’s dirty plans started to unravel.
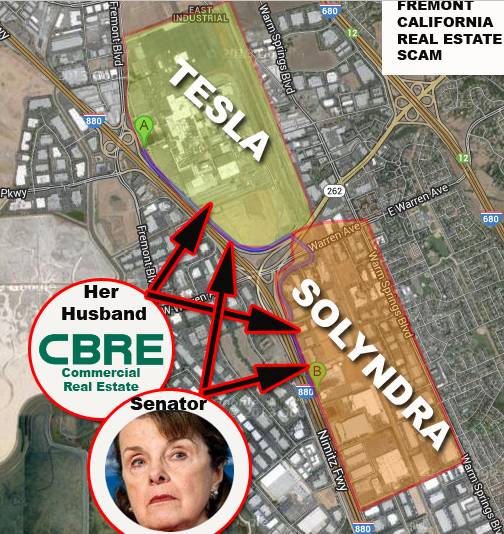
Was the death of Gary D. Conley, the Cleantech CEO found with a bullet in his head, in the scrub brush behind the Air Force base, also connected to this? Conley blew the whistle on side-by-side federal funding scammers Solyndra (Raided by the FBI) and Tesla.
There are quite a large number of beat-up, harassed and dead bodies in this tale!
To understand why a “green car company” might go to such lengths, you need to go to the drug-store and buy a big sponge and a little bottle of black India ink.
Get a one inch, or thicker, sponge that is super dry, (Don’t use one of those slightly moist kinds). Try to find one about 4 inches by 6 inches.
Now take a dinner plate and pour about a quarter inch of ink on it.
Now drop the perfectly dry sponge on top of the ink and OBSERVE!

The black stain starts at the bottom of the sponge and creeps UP, through the whole sponge, until the entire sponge is black. That was interesting but…wait, something isn’t right, you say to yourself…GRAVITY! Huh?
Yes, you just watched something seem to “defy gravity”. A material, on it’s own, seemed to go up, and left and right, against the flow of gravity. But, wait, that’s not what you were taught in school!
That plate full of black goo is the Tesla Gigafactory and it’s run-off, that dry sponge 500 miles of the dry desert soil of Nevada. The black goo, itself, is one of the most lethal cocktails of chemicals that mankind has ever attempted to commercialize. You just watched a nightmare unfold. That is the secret buried deep in the Nevada desert. It isn’t aliens and Area 51. It is toxic hell!
The reason the Tesla Gigafactory is hidden behind sand-swept berms in the desert is because they don’t want you to notice it. They want it to deliver it’s toxic package to the soil, air and workers of Nevada in a tidy, hidden package.
Those toxins can move through hundreds and hundreds of miles of the air.
Those toxins can move DOWN through miles and miles of soil and then SIDEWAYS, in every direction, for over 500 miles! Yes, solid soil has toxic rivers that creep through it, and never stop spreading. Tesla is a Death Factory.
In China and other parts of Asia, the same kind of battery factories have killed thousands of workers, poisoned hundreds of towns and inflicted tens of thousands of people with deadly diseases and mutated babies. Elon Musk and Harry Reid knew that these dangers existed by they wanted the tens of millions of dollars in profit, that they plan to make off of this factory.
All of the current Tesla batteries and every future planned Tesla batteries using chemicals which are proven, without question, and by the U.S. Government, to be deadly. When mixed together they become worse. While mixing them together, as a worker, they kill you in an amazing number of ways. This is not speculation. Read the federal MSDS sheets on each chemical and combination of chemicals.
Look at what happens to a worker even 100 feet from the dust created by the powders used in the batteries. Those powders can travel to Reno and Las Vegas, on the wind, in minutes.
Elon Musk says: “Don’t worry”, walk away, nothing to see here. He says that the only immediate neighbors are “funky whorehouses”, why worry? Know will care about a few poisoned hookers. He lies.
The toxic air and water from the Tesla Gigafactory will reach all of Nevada and Utah and California and keep on going.
Green advocates wring their hands at “Climate deniers” and cry that “Those who ignore scientific facts and historical documentation of damage to humans should be put in jail!”
In the case of the Tesla Gigafactory, The U.S. Government, and every major University has released deeply peer-reviewed scientific proof that Tesla’s battery chemicals explode, cause fires, kill, cause cancer, kill towns, poison crops, travel vast distances in the air and soil, cause brain damage, cause liver damage and mutate the fetus. The indisputable historical facts about these factories in China and Southeast Asia has proven that those dangers always happen.
The scientific proof is rock-solid. To allow the corrupt Tesla operation to continue is a crime against humanity. Elon Musk, and his partners, may even kill to hide this trillion dollar lethal secret.
The Technical Background:
Let’s begin by looking closely at the soil. Because chemical transport, interactions and transformations occur in soil, soil composition is important in water and chemical movement. Soils are composed of three phases; solid, liquid and gas. The solid phase includes primary particles of sand, silt, and clay, organic matter, and rocks and minerals too large to be classified as sand. Soil water is the liquid phase, and air is the gaseous phase. Water and air fill the void space in soils. The amount of air and water in the void space influences microbial activity, water movement and chemical movement in soils. In saturated soils, the void space is filled with water.
Although saturated flow conditions may occur, under prolonged saturation, anaerobic processes begin to prevail. As water cycles through the environment, it carries dissolved Tesla chemicals. Water movement is generally the most important process that transports chemicals through soil.
Tesla’s manufacturing activities contribute to chemicals in water and soils. These chemicals may come from Tesla’s cars, factory transport trucks, applied compounds, waste products, or accidental spills. Specific examples include sewage, wastes, cleaning processing wastes, industrial chemicals, dry cleaning solvents, landfill leachate, fuel, motor oil and factory equipment cleaning products. Chemicals are seldom put in water to intentionally degrade water quality. Rather, as water moves from the soil surface to groundwater (or surface water bodies) it contacts chemicals in the soil and dissolves some of them.
The water carries those dissolved chemicals with it as it moves. The major processes that move chemicals through soil are diffusion, convection and hydrodynamic dispersion.
Diffusion
Diffusion is the movement of Tesla’s toxic chemicals from areas of high concentration to areas of low concentration. Diffusion occurs due to the random movement of chemical molecules. This motion is due to nonuniform, random collisions of molecules. An example may help us visualize this concept. The billiard balls act as individual molecules would, by distributing themselves more evenly within the available space.
Since the number of collisions tends to be greater where many billiard balls are located, the collisions tend to move a ball away from other balls. Similarly, molecular collisions result in molecules moving from regions of high concentrations to regions of low concentrations. Compared with other transport processes, diffusion is a relatively slow process. You can see an example of gaseous diffusion utilizing a glass tube with cotton batting stuffed in both ends. Hydrochloric acid is added to one end of the tube, while the opposite end receives ammonium hydroxide. Both substances produce gases that diffuse from the ends of the tube toward the middle. Where the two gases meet, they react chemically producing ammonium chloride, visible as the white powder being formed at the location where the gases meet. Because diffusion is slow compared to chemical transport in convecting water, diffusion is not readily apparent when viewing water and chemicals moving through soil.
Convection
Convection is fluid motion caused by external forces. An example of convection is water flowing along a stream bed. This flow occurs when water moves from higher elevation to lower elevation. This flow is due to a difference in energy levels at the two elevations. Water at the higher elevation (point A) has a greater potential energy than water at the lower elevation (point B). This potential energy difference causes the water to move from point A to point B. When the potential energy difference is large and occurs over a short distance, the water moves quickly. We see this in rapidly flowing surface runoff water, streams and waterfalls. When the gradient of the soil surface elevation is small, the flow of water down a stream is fairly slow. Movement of water and chemicals in soil occurs due to differences in the potential energy of water in the soil. The potential energy level is usually due to gravity and attractive forces associated with small pores between soil particles.
In a demonstration, the potential energy of soil water is much larger in the wet soil near the soil surface than it is in the dry soil below. As infiltration begins, the distance between the soil surface and
underlying dry soil is small, so the soil water potential energy gradient is relatively large. Consequently, water moves fairly rapidly into and through the soil. Later, when the same soil is wetted to a much deeper depth, the distance between the soil surface and underlying dry soil is larger. The soil water potential gradient is now much smaller. Consequently, water moves into and through the soil much more slowly.
Whether it moves rapidly or slowly, this flow of soil water is called convection. The transport of chemicals in soil water is called advection.
Hydrodynamic Dispersion
When water moves through soil, it travels around soil particles and rocks, following flow paths that
act like a bundle of capillary tubes of different lengths. Water and chemicals following these tortuous paths create a phenomenon called hydrodynamic dispersion. (1) Two water molecules may follow different flow paths, so the actual distances they travel may be quite different. So, they may arrive at the same destination at substantially different times. (2) Since the actual water flow paths in most soils must curve around solid soil particles and air space, water and dissolved chemicals follow a tortuous path. This demonstration helps us see how the length of the flow path affects the arrival time of water and chemicals. The two tubes represent two different flow paths water may take when leaving point (A). Both tubes begin at the same point, but one is fairly short, and the water leaving (A) arrives at (B) quickly. The second tube curves frequently, creating a tortuus path for the water to follow. Consequently, the distance water must travel to arrive at point B is greater if it travels through the tube
on the left. Saturated flow through soil is similar to flow through different length tubes. Therefore, chemicals entering the soil at the same time arrive at a given depth at different times. When a chemical first appears at a point below the soil surface, its concentration in the soil water will be less than the concentration at which the chemical was first applied. This is because of dilution, which occurs independently from any interaction of the chemical with soil particles.
This model helps us begin to understand some of the important concepts of how water and chemicals
move through soil. Water movement in real soil is not so ideal, however. Soils are not uniform in texture or structure; or in the distribution of their organic matter. Some pore spaces between soil particles may be blocked, SLOWING water and chemical movement. Large cracks, animal burrows and former root channels may exist which allow rapid movement of water and dissolved chemicals.
When water and dissolved chemicals move in a non-uniform manner through soil, the movement is often called preferential flow. Soil layers of differing textures and densities can also cause the flow of water and chemicals to vary.
Interactions
Chemical characteristics influence the ability of substances to be transported. Characteristics of particu-
lar interest include solubility, sorption and density. Chemicals that are more soluble at the soil’s pH tend to move more easily with water than chemicals that are less water soluble. In contrast, chemicals with lower water solubilities will tend to attach to clay particles and organic matter near the soil surface. Some of these will form chemical precipitates. If soil particles are moved by water or wind erosion, attached chemicals will move with them. In this way, chemicals are carried across the soil surface away from their point of application, and sometimes into surface water. Chemicals that are only slightly water soluble can still reach surface or ground waters. However, their rates of movement will tend to be slowed through interactions with soil particles.
Adsorption often refers to the process where molecules are attracted to the surface of soil particles.
True adsorption occurs when molecular layers form on a soil particle surface. When molecules commingle with another substance, we refer to the process as absorption. Most soils absorb water and chemicals, although in amounts much less than those adsorbed. In practice, it is difficult to distinguish between absorption, adsorption and other processes. Desorption is the process by which molecules are
detached from the surface of soil particles. Adsorption and desorption usually occur simultaneously. Molecules and ions are continually transferred between the soil solution and soil particle surface.
Since the specific process is difficult to measure, the more general term, sorption, can be used to describe how a chemical is held in the soil. Clay particles and organic matter are the most chemically active soil solids. They are the major soil components to which chemicals sorb. Most chemicals are subject to forces of sorption. Examples include simple inorganic ions such as calcium, sodium, and ammonium. Complex organic chemicals such as humus, many pesticides and industrial solvents are also sorbed onto soil. Chemical
s such as phosphorus, and similar Tesla chemicals, that are strongly
sorbed to soil particles near the surface of most soils will tend to contaminate surface water if erosion is a problem.
Chemicals, such as nitrate, that are more water soluble and less strongly sorbed to soil particles, will
tend to contaminate ground water if rainfall or irrigation exceeds plant water use. Positively charged ions (called cations) are attracted to a negatively charged site on clay or organic particles. The movement of cations between clay or organic particles and the surrounding soil water is called cation exchange. It is an important process. It controls the mobility of many chemicals through the soil profile. Cation exchange is seldom observed with most organic chemicals that might be added to the soil, because few organic chemicals carry positive charges at a normal soil pH. However, some examples do exist. The pesticides Paraquat and Diquat are examples of cationic pesticides that can be sorbed onto soil particles through cation exchange. A number of other bonding mechanisms exist by
which organic compounds are sorbed to soil surfaces.
For any given Tesla manufacturing or cleaning compound (organic or inorganic), it is likely that a combination of mechanisms is responsible for sorption onto soil. Whatever the mechanism, soil organic matter is the principal sorbent for many nonionic organic chemicals. It is important to know a particular chemical’s attraction to organic matter, and the amount of organic matter available in a particular soil.
Then one can estimate the leaching potential of various chemicals used in a management system.
Many demonstrations show two chemicals, one is sorbed to the soil, the other is not. A yellow solution, like nitrate, is not sorbed to the soil, while a red, like ammonium, is sorbed. Because it is not
sorbed, the yellow solution reaches the bottom of the soil column fairly rapidly, while the red solution is sorbed to the soil surface, restricting its downward movement. It should be noted that while the soil retains red solution, the soil does not prevent it from moving downward. The soil merely slows the rate of the red dye movement, relative to the rate of the water movement. Obviously, the same amount of water moving through the soil would affect the depth of movement of these two chemicals quite differently. Chemicals applied to land surfaces are not the only source of contaminants affecting ground and surface waters. Fluids that leak from underground storage tanks can also move to ground water, and can move to nearby surface water. Frequently such chemicals do not mix with water. Their transport is less predictable than transport of chemicals that are more soluble in water. One interesting aspect of chemical transport involves whether chemicals are more or less dense than water. This demonstration shows a chemical developing fingers because it is more dense than water. These fingers of concentrated chemical sink to the bottom of the water column before they appreciably mix with the water. Spilled chemicals that are more dense than water will tend to sink to the lower depths of a ground water aquifer.
Chemicals that are less dense than water (for example, gasoline) will tend to float near the top of a
ground water aquifer. Without significant mixing due to groundwater movement, chemicals that are ap-
proximately the same density as water tend to remain near the top of a groundwater aquifer. So, chemical sorption to soil particles, chemical solubility and chemical density all affect the rate of
chemical transport.
Transformation
Tesla chemicals undergo numerous transformations in both soil and water. Hydrolysis, photolysis, oxidation, and reduction are some of the most common transformations. Hydrolysis is the cleavage of molecules by water, and is one of the most important reactions in breaking down pesticides. Hydrolysis can occur in the soil with or without microorganisms. Photolysis is the process where ultraviolet or visible light supplies the energy for decomposition of chemical compounds. Photolysis can be a very important chemical transformation process. Oxidation is the process where a chemical loses
electrons, such as rust forming on iron. Reduction is the process where a chemical gains electrons. Reduction can be a non-biological process, or a biological process as in anaerobic sewage treatment.
The transformation frequently simplifies the chemical nature of the substance. Degradates or metabolites are the terms used for products transformed from the original chemical.
These products may be sorbed to soil more or less strongly than the original compounds. They may
also be more or less hazardous than the original compound. For example, Malathion is an organophosphate insecticide commonly used both in agriculture and in and around commercial and private residences. Soil bacteria may chemically convert Malathion into a closely related compound called Malaoxon, which is more toxic than the Malathion itself.
One demonstration shows how chemical transformations can occur in soils. The rate at which such
transformation occurs depends upon the location of the chemical in the soil. If a transformation is biological, and it is enhanced by aerobic conditions, it is likely to occur more rapidly near the soil surface. The soil surface has more nutrients and oxygen available for microorganisms to grow. One demonstration also shows that two different chemicals, represented by red and yellow colors, may be transformed at different rates. The red dye is rapidly degraded and becomes colorless, while the yellow dye is only slightly degraded during this demonstration. For transformations that involve organic chemicals, such as most pesticides, we use the term half-life in discussing the rate of transformation. This simply describes the length of time required to transform 50% of the existing chemical. The amount of chemical remaining reflects an exponential decrease over time, since after one half-life time period, 50% of the original chemical remains. After two half-life time periods, 25% of the original chemical remains. After three half-life time periods, 12.5% of the original chemical remains, and so forth. The “model” used to describe the disappearance of the original chemical over time creates the perception that: (1) the chemical never is completely transformed, and (2) the transformation rate is well defined. Neither is completely true, although experimental data are often reasonably well described by this exponential decay model.
The combined effects of water movement, soil interaction and transformations determine chemical concentrations below the root zone. Let’s look at examples of two toxic Tesla chemicals as they move through soil. In the first example, a chemical that is strongly sorbed to a soil is compared with one that is only moderately sorbed. We assume rainfall or irrigation exceeds crop water use, for at least some of the days during the growing season. We also assume the half-life of each chemical is the same and the water table is well below the root zone of the crop. The moderately sorbed chemical moves noticeably
deeper into the soil profile after rainfall. The strongly sorbed chemical moves much more slowly. Half way through the season, the moderately sorbed chemical is below the active root zone of the crop, while the strongly sorbed chemical is still near the soil surface. The strongly sorbed chemical is less likely to contaminate the ground water. However, if substantial soil erosion occurs, we would expect to find the strongly sorbed chemical and sediment in ne
arby surface water. Depending upon the depth to ground-water, the moderately sorbed chemical may be degraded before reaching the water table. Con-
versely, if the water table is near the bottom of the root zone, it is likely that the moderately sorbed
chemical will find its way into ground water before it is completely degraded. The second example includes two chemicals that are moderately sorbed to soil. The first chemical has a relatively long half life; the second chemical has a short half life. When they move below the root zone near mid-season, the first chemical is still present in fairly high concentrations. The second chemical with the shorter half-life is present in low concentrations. The chemical fronts tend to broaden as they move downward. This is due primarily to hydrodynamic dispersion, although some diffusion also occurs. Hydrodynamic dispersion spreads out the chemical and reduces its maximum concentration at a particular point in the soil.
Summary:
Summary Point #1: The concepts of diffusion, convection, and hydrodynamic dispersion relate to the transport of Tesla’s toxic chemicals through the soil.
Summary Point #2: Solubility, sorption and density are the characteristics that have the most influence on the way toxic Tesla chemical substances interact with soil.
Summary Point #3: As toxic Tesla chemicals are transported through soil, they can be altered by biological or chemical processes, or remain relatively unchanged.
Summary Point #4: Transformations may form new substances which may be more or less environmentally hazardous than the original Tesla chemical. Transformations may also produce substances that have different characteristics than the original chemical. These characteristics may affect the ability of the substance to be sorbed, degraded or dissolved. Tesla has no plans, systems or technology planned, or in place, to measure these chemical changes between their factories and the borders of various cities and states
It is a total lie, by Elon Musk, for him to say that the poisons from the Tesla factory will not soon end up in every structure in Las Vegas, with a concrete foundation. Concrete is a sponge. While Harry Reid, Dianne Feinstein and Elon Musk, who all own stock and other financial assets in this, and related ventures, would like these facts covered up, they are now available for your introspection.
How To Document These Dangers:
Previously- You needed to hire a deep drilling truck, an entire testing lab and drill out a grid across many miles of land at massive expense per drill site. That is no longer needed. You can now test counties and neighborhoods with handheld test devices that are far less expensive. Many groups buy their own systems and have parents swap neighborhood testing duties supervised by a technical aid.
Devices to self test include, but are not limited to:
http://cwmi.css.cornell.edu/soilquality.htm
http://www.sciencedaily.com/releases/2010/06/100609201310.htm
and many hundreds of other devices.
Once generalized testing has targeted problrms, limited testing can be conducted and the test cores can be tested on-site with the hand-held devices.
Many outsourced testing companies are controlled by your opposition, so be careful. Try to do it all yourself and control everything.